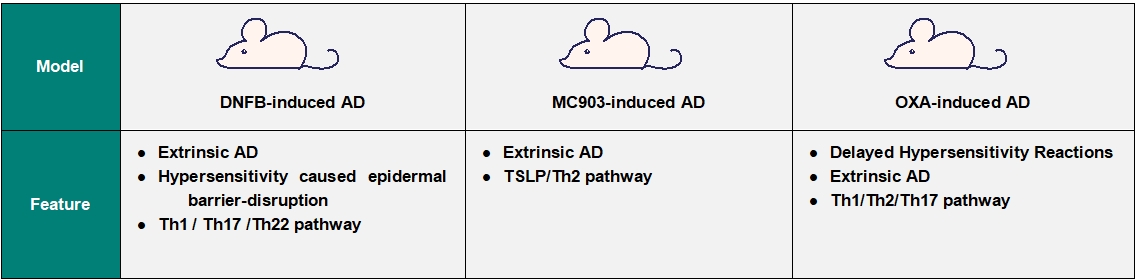
GemPharmatech has developed several atopic dermatitis mouse models that replicate human pathology, including IgE evaluation, chronic pruritus, epidermal hyperplasia, and immune cell infiltration. Our models ensure clinically translatable efficacy data for diverse drug candidates: Th2 pathway modulators, JAK inhibitors, biologics, and more. Endpoints include histopathological scoring, cytokine profiling, and molecular biomarker analysis. There are three validated AD models with distinct mechanistic profiles:
DNFB induced AD
The DNFB-induced atopic dermatitis (AD) mouse model is uniquely constructed to exhibit robust pruritus (itch) phenotypes for enhanced therapeutic evaluation. Our models recapitulate key clinical hallmarks, including intense scratching behavior, epidermal thickening, and Th2-skewed immune responses, ensuring translational relevance for anti-itch drug development.
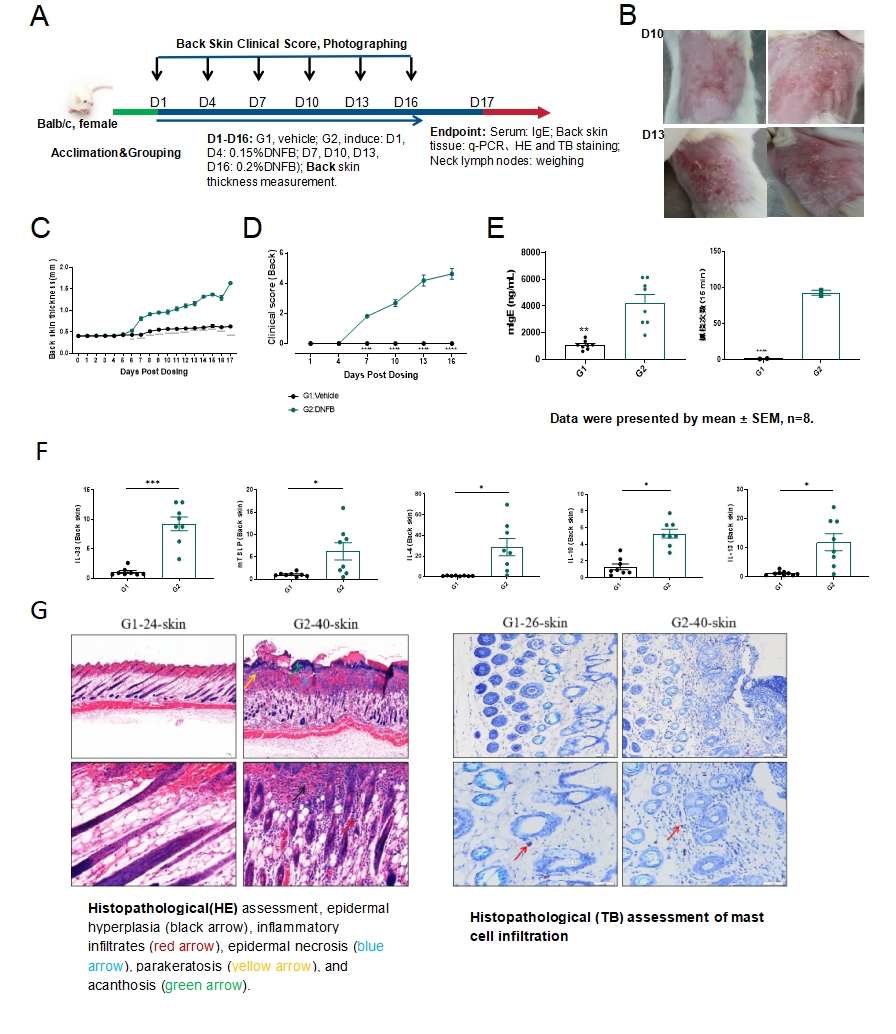
Fig.1 DNFB-induced mouse Atopic Dermatitis model
The model strategy of DNFB-induced atopic dermatitis is shown in Fig.1A. After DNFB induction for 16 days, the representative photos of back skin were shown (Fig.1B). The thickness and clinical score of back skin increased significantly in the model group (Fig.1C&1D), with an accompanying increase in IgE serum levels and scratching frequency in mice after DNFB induction(Fig.1E). The mRNA expression level of type 2 cytokines including IL-33, IL-4, IL-13 and TSLP in the back skin increased significantly (Fig.1F). The results of HE staining and TB(Toluidine Blue) staining of the skin showed inflammatory cells and mast cells infiltration in the model group (Fig.1G). Data were presented by mean ± SEM. *P<0.05,**P<0.01;***P<0.001.
MC903-induced atopic dermatitis
The MC903-induced AD mouse model is a premier preclinical tool for evaluating therapeutics focused on the TSLP pathway. By stimulating keratinocyte-derived TSLP, this model drives AD-like inflammation through ILC2 activation and robust Th2 cytokine production (e.g., IL-4, IL-13), closely mirroring human AD pathogenesis. Its TSLP-dependent mechanism ensures high specificity for testing large-molecule drugs, including monoclonal antibodies or biologics targeting TSLP or downstream immune signaling.
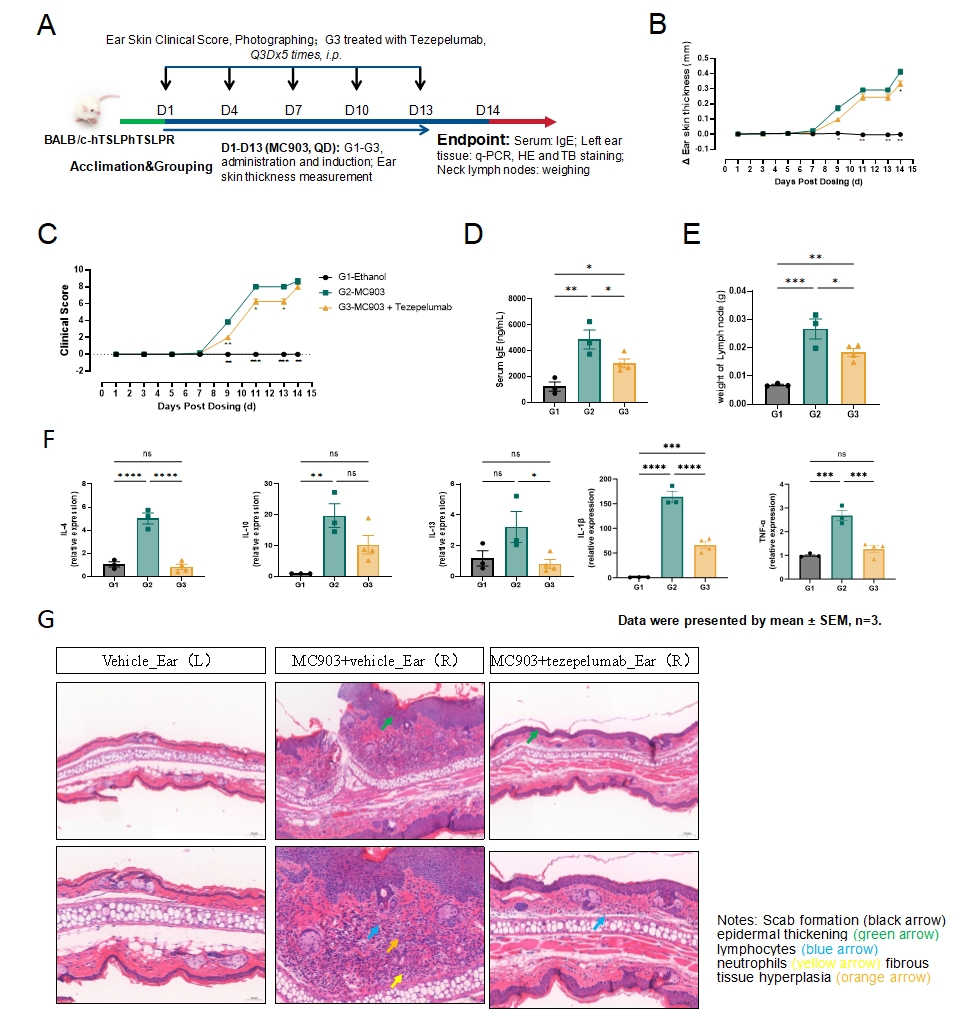
Fig.2 MC903-induced mouse atopic dermatitis model
The model strategy of MC903-induced atopic dermatitis is shown in Fig.2A. The thickness and clinical score of ear skin increased significantly in the model group which decreased after Tezepelumab treatment (Fig.2B&2C). The level of IgE in the serum and lymph node weight of mice increased significantly after MC903 induction and significantly decreased after Tezepelumab treatment(Fig.2D&2E). The mRNA expression level of IL-4, IL-1β and TNF-α in the ear skin increased significantly, and significantly decreased after Tezepelumab treatment(Fig.2F). The results of HE staining of the ear skin showed inflammatory cell infiltration and epidermal thickening in the model group, which was inhibited by Tezepelumab treatment (Fig.2G).Data were presented by mean ± SEM. *P<0.05,**P<0.01;***P<0.001.
OXA-induced atopic dermatitis
Oxazolone (OXA) triggers AD-like lesions characterized by epidermal thickening, immune cell infiltration, and elevated Th2/Th1 cytokine levels (e.g., IL-4, IL-13, IFN-γ), mimicking key aspects of human AD. This model emphasizes T-cell-mediated hypersensitivity and chronic inflammation, making it ideal for testing immunomodulators, JAK inhibitors, or biologics addressing multi-cytokine pathways. With high reproducibility and clinical translatability, the OXA model provides actionable insights into drug efficacy, itch modulation, and skin barrier restoration. Its adaptability to acute/chronic phases allows tailored study designs for target validation and dose optimization.
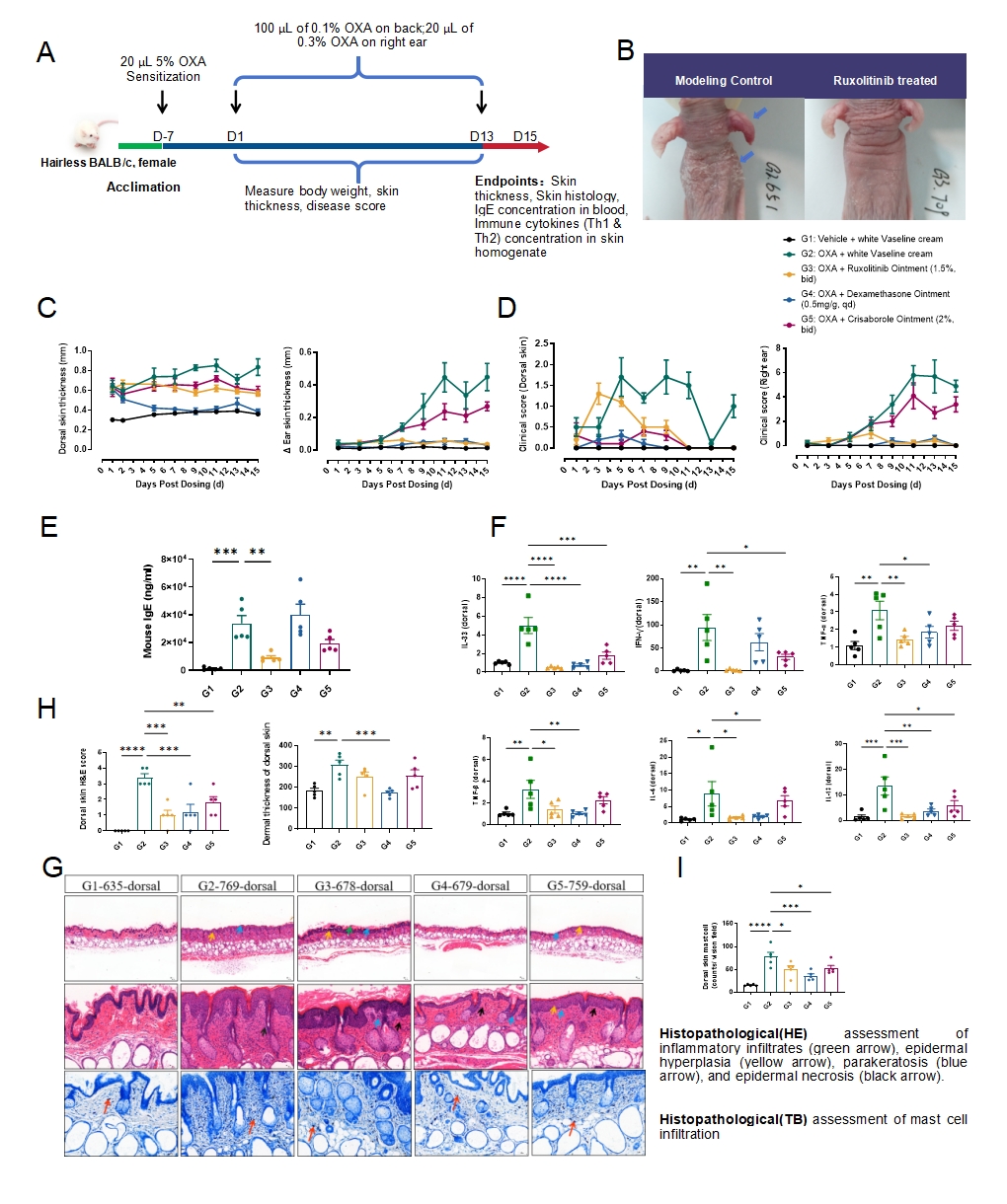
Fig.3 OXA-induced mouse atopic dermatitis model
The model strategy of OXA-induced atopic dermatitis is shown in Fig.3A. After OXA induction for 13 days, the representative photos of back and ear skin were shown (Fig.3B). The thickness and clinical score of ear skin increased significantly in the model group, and decreased with Ruxolitinib, dexamethasone or Crisaborole treatment (Fig.3C&3D). The level of IgE in the serum of mice increased significantly after OXA induction and decreased with Ruxolitinib treatment (Fig.3E). The mRNA expression level of IL-33, IL-4, IL-13, IFN-γ, TNF-β and TNF-α in the ear skin increased and decreased with Ruxolitinib treatment(Fig.3F). The results of HE and TB staining of the dorsal skin showed inflammatory cells, mast cells infiltration and epidermal thickening in the model group, which was inhibited by Ruxolitinib or dexamethasone treatment (Fig.3G&3H&3I).Data were presented by mean ± SEM, n=5.*P<0.05,**P<0.01;***P<0.001.****P<0.0001.