Myocardial Infarction (MI) is caused by acute coronary artery occlusion, leading to myocardial ischemia, necrosis, and impaired cardiac function, whereas acute heart failure (AHF) manifests as rapid-onset symptoms (e.g., dyspnea, edema) due to cardiac dysfunction, often with elevated natriuretic peptides. The mouse left anterior descending (LAD) coronary artery ligation model mimics human MI by inducing localized ischemia and myocardial cell death. This triggers ventricular remodeling, including chamber dilation, wall thinning, and systolic dysfunction, which progress to heart failure characterized by reduced ejection fraction and fibrosis. This model replicates both acute ischemic injury (MI phase) and chronic decompensated cardiac dysfunction (AHF phase), enabling mechanistic and therapeutic studies.
This mouse left anterior descending (LAD) coronary artery ligation model induces myocardial infarction via minimally invasive thoracic access, achieving rapid LAD occlusion without ventilator support. This method reduces surgical trauma and mortality, reliably causing cardiac ischemia, fibrosis, and functional decline, validated by pathological staining and echocardiography
Example Data
STEMI ECG well identified in LAD model
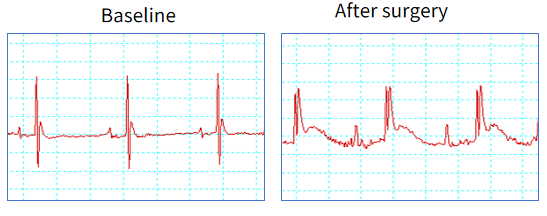
Figure 1. The murine left anterior descending (LAD) coronary artery ligation model accurately recapitulates ST-segment elevation myocardial infarction (STEMI) pathophysiology, with characteristic ECG features including ST-segment elevation.
LAD results in transient decrease of body weight
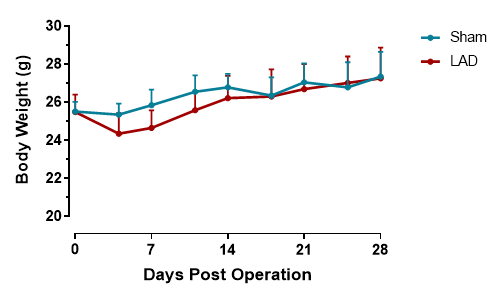
Figure 2. Body weight after LAD. LAD ligation surgery induces a transient body weight reduction during the first postoperative week, likely due to surgical stress and reduced feeding activity. Body weight typically normalizes by week 2-3, confirming the acute nature of this metabolic perturbation. n=11, Data are presented as Mean ± SD.
Mortality of partial LAD mice in 1st week after surgery
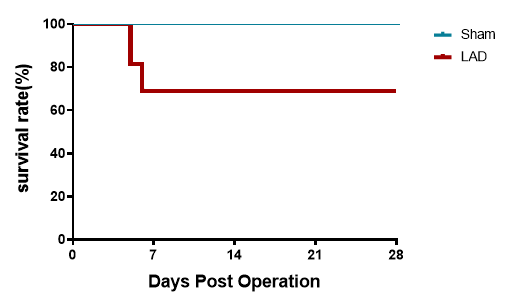
Figure 3. Survival curve after LAD. The Kaplan-Meier curve demonstrates a steep survival decline in LAD-operated mice (28-day survival: 60-70%) versus 100% survival in sham controls. Acute mortality (Days 0-7) primarily stems from cardiac rupture and hemorrhage, reflecting perioperative ischemic ventricular wall fragility. Survival stabilizes post-Day 7 as surviving mice overcome acute mechanical complications. n=11.
LAD induced ischemia results systolic dysfunction 1 week after surgery
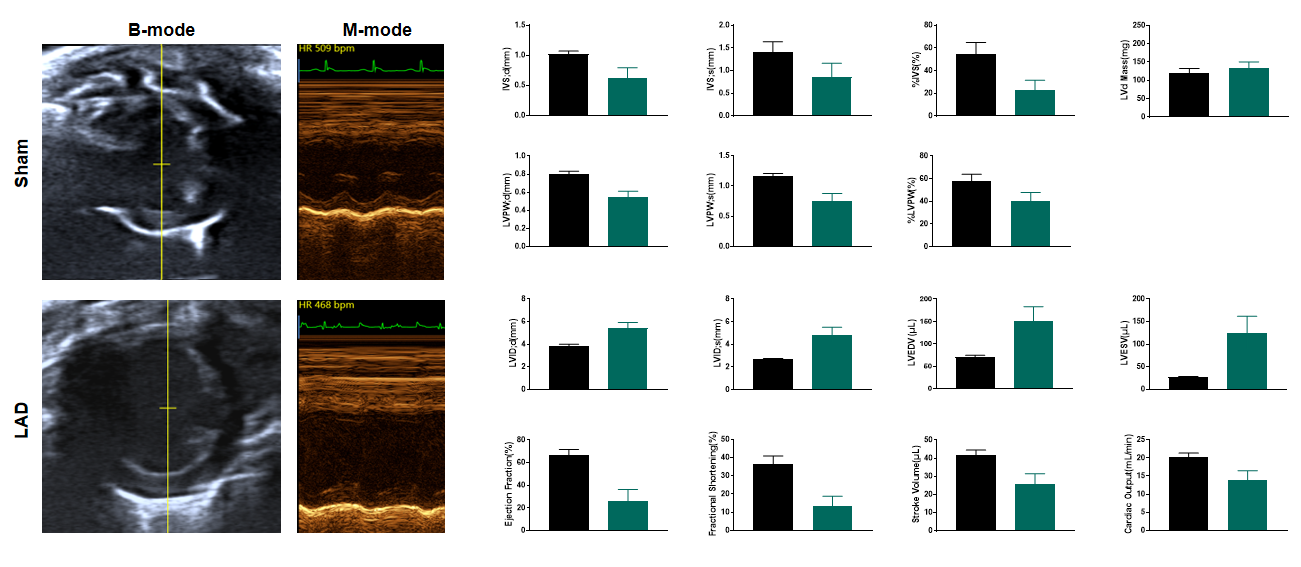
Figure 4. Echocardiography in PSAX 1 week after LAD. Post-LAD echocardiography reveals severe systolic dysfunction in the operated group, evidenced by markedly reduced left ventricular ejection fraction (LVEF) and fractional shortening (FS) compared to baseline. The B-mode images demonstrate ventricular wall thinning and chamber dilation in the ischemic anterior wall, while M-mode tracing shows diminished septal wall motion amplitude (≤1.0 mm vs. normal >2.5 mm). These findings confirm pathological ventricular remodeling, including impaired contractility and adverse geometric changes, consistent with ischemic myocardial injury progressing toward heart failure. n=11, Data presented as Mean ± SEM.
LAD induced ischemia results systolic dysfunction 2 weeks after surgery
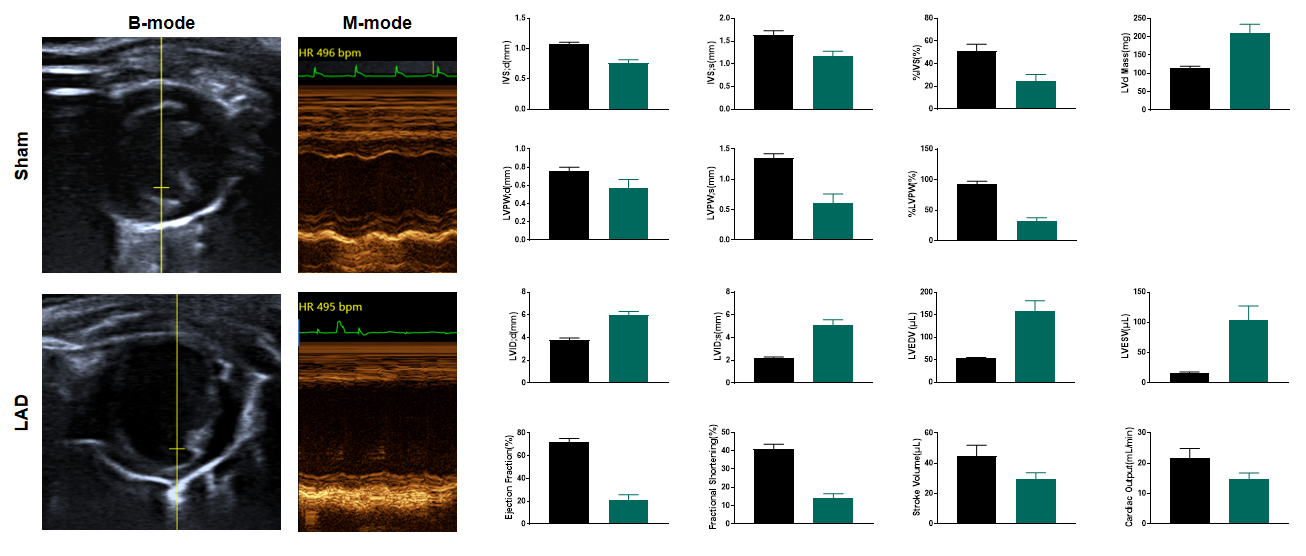
Figure 5. Echocardiography in PSAX 2 weeks after LAD. Echocardiography at 2 weeks post-LAD ligation reveals severe left ventricular systolic dysfunction, with ejection fraction (EF) and fractional shortening (FS) reduced by ~50% compared to sham controls. M-mode tracing demonstrates anterior wall hypokinesis and paradoxical septal motion, while B-mode images exhibit dilated LV chambers and wall thinning at the infarct border zone. These findings confirm ischemic cardiomyopathy progression. n=11, Data presented as Mean ± SEM.
LAD results in advanced heart failure 4 weeks after surgery
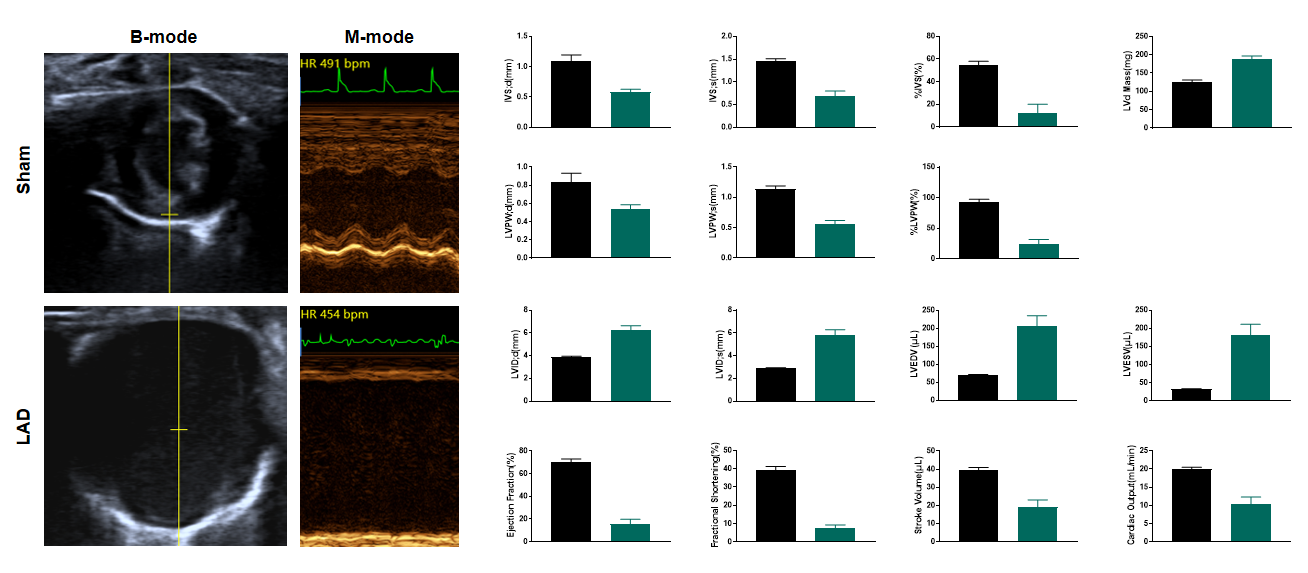
Figure 6. Echocardiography in PSAX 4 weeks after LAD. Echocardiography at 4 weeks post-LAD ligation reveals severe systolic dysfunction, with ejection fraction (EF) plummeting below 20% (vs. ~65% in Sham, p<0.001). B-mode imaging demonstrates pronounced left ventricular dilation (LVEDV↑ >100%) and anterior wall akinesis, while M-mode tracing shows near-absent septal wall motion. These findings confirm advanced ischemic cardiomyopathy with adverse ventricular remodeling, consistent with large transmural infarction. n=11, Data presented as Mean ± SEM.
Heart failure with large transmural infarction 4 weeks after LAD
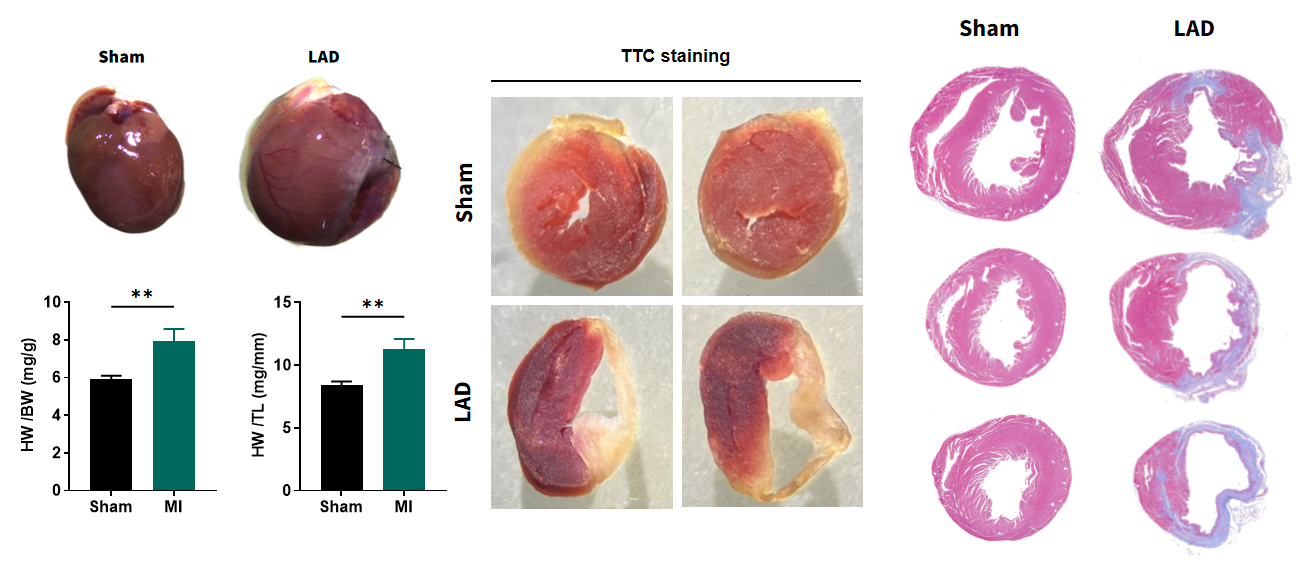
Figure 7. Pathological analysis of LAD hearts 4 weeks after surgery. Four weeks post-LAD ligation, myocardial infarction (MI) mice exhibit significant cardiac hypertrophy, with elevated heart weight-to-body weight and heart weight-to-tibia length ratios, indicating compensatory ventricular remodeling. TTC staining confirms extensive infarction in LAD hearts, contrasting with intact Sham myocardium. Histological sections reveal fibrotic scar consistent with ischemic injury progression. These structural alterations correlate with functional decline demonstrating successful modeling of post-infarct pathological fibrotic remodeling. n=11, Data presented as Mean ± SEM. **, p<0.01 by t-test.
Typical Study design
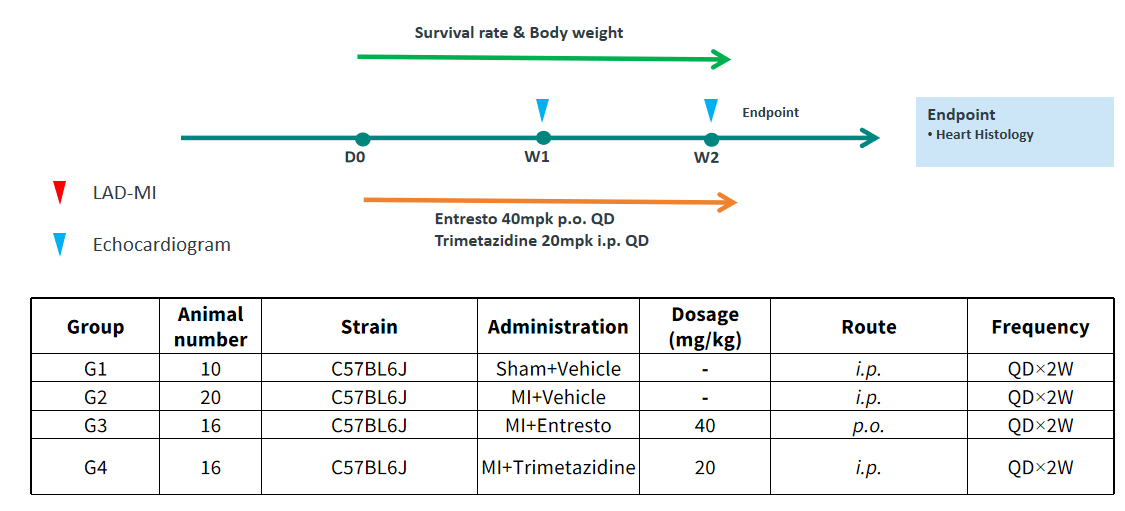
Figure 8. Study designs.
Entresto evaluates survival rate in following 1 week post LAD
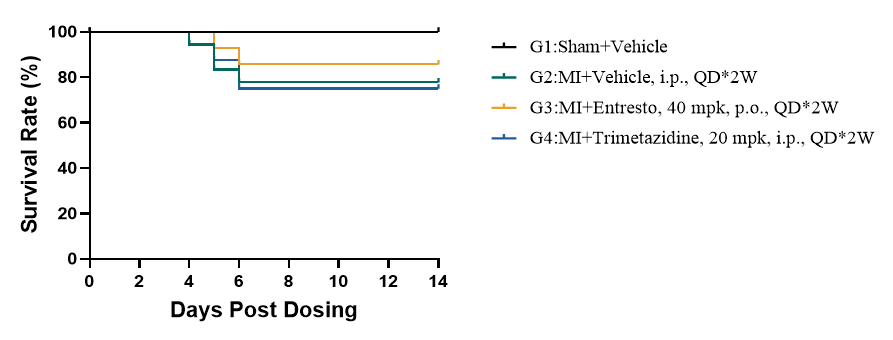
Figure 9. Kaplan-Meier survival curve. All mortality is observed within the first week post-LAD, aligning with typical pos-MI complications like cardiac rupture. G3 (Entresto) showed moderate attrition: G2 (MI+Vehicle) at 80-90%, and MI groups (G2) G4 (Trimetazidine) at ~60-80%, suggesting therapeutic benefit from Entresto in reducing acute mortality. n=10.
Entresto prevents cardiac remolding and sustains cardiac function
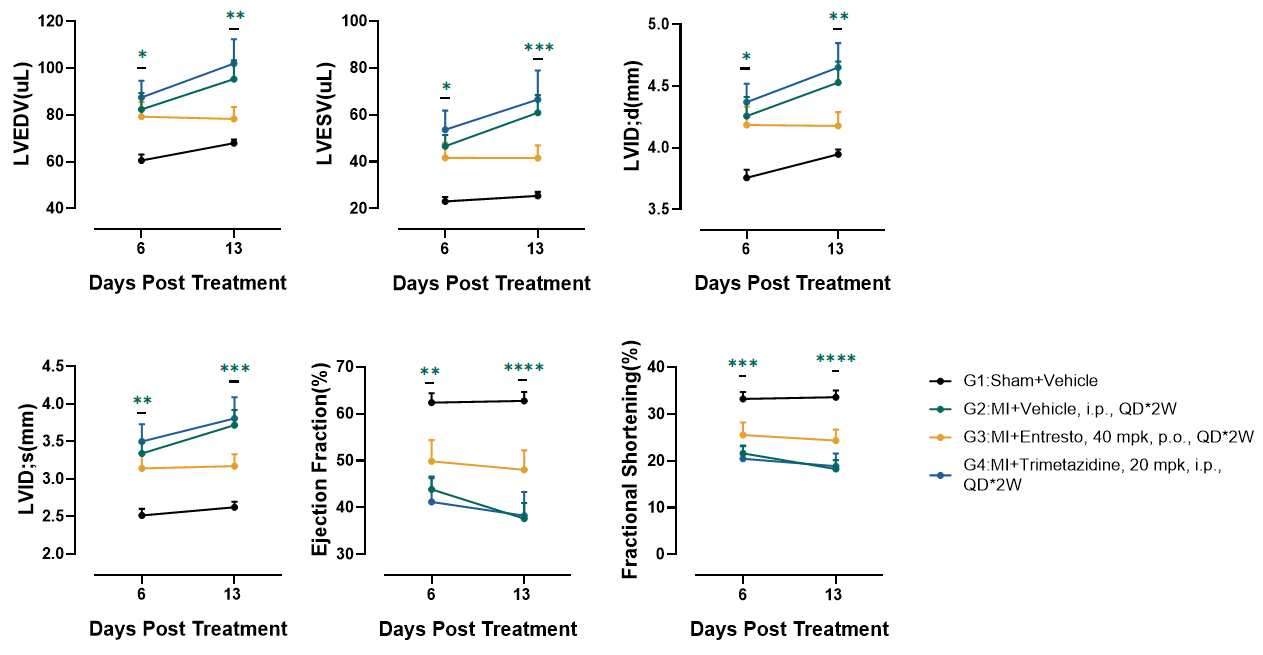
Figure 10. Echocardiography in PSAX. At 4 weeks post-LAD (Day 13), MI+Vehicle (G2) exhibited severe systolic dysfunction with ejection fraction (EF) and fractional shortening (FS). Ventricular dilation was evident in G2: LVEDV and LVESV increased significantly, confirming pathological remodeling. Entresto (G3) partially attenuated dysfunction, suggesting Entresto's dual neprilysin/RAAS inhibition may mitigate adverse remodeling. Trimetazidine (G4) showed no benefit which may be due to drug delivery method. n=10, Data presented as Mean ± SEM. *, p<0.05; **, p<0.01; ***, p<0.001;****, p<0.0001 by one-way ANOVA with Dunnett’s pot-hoc test.