High fat diet pulsed L-NAME (HFD+L-NAME)-induced heart failure with preserved ejection fraction (HFpEF)
Heart failure with preserved ejection fraction (HFpEF) is characterized by diastolic dysfunction, myocardial stiffness, and comorbidities like obesity, hypertension, and metabolic syndrome. The HFD+L-NAME-induced mouse model combines high-fat diet (HFD, 60% kcal fat) to mimic metabolic stress and L-NAME (a nitric oxide synthase inhibitor) to induce hypertension and vascular dysfunction. This dual-hit model recapitulates key HFpEF features: preserved LV ejection fraction, diastolic impairment (elevated E/E' ratio), myocardial hypertrophy, fibrosis, pulmonary congestion, and reduced exercise tolerance, mirroring human HFpEF pathophysiology.
Study Design and Example Data
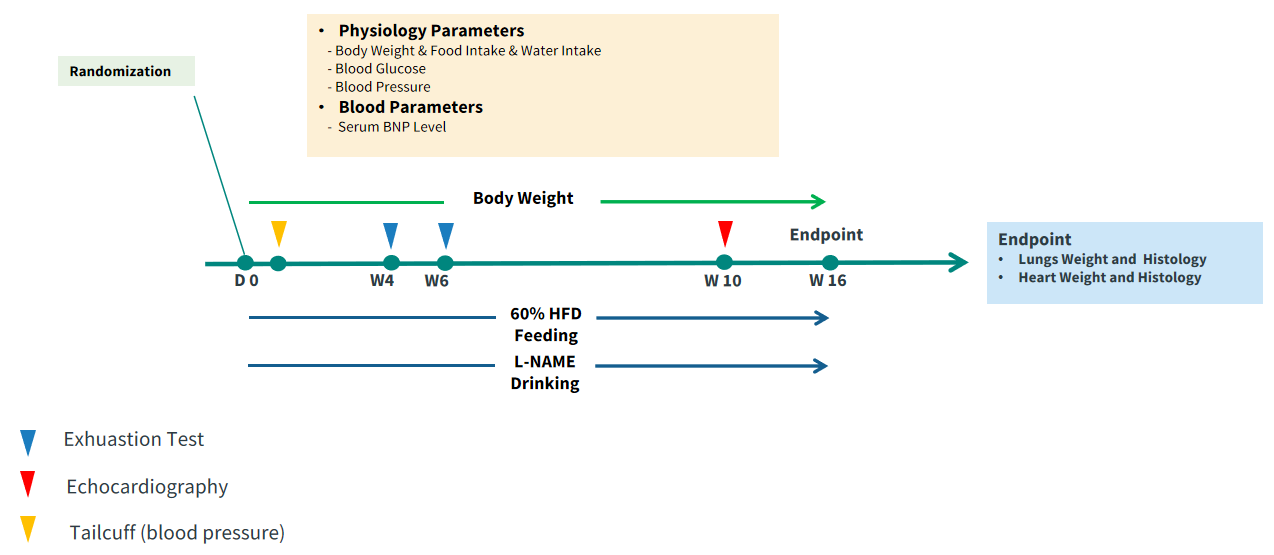
Figure 1. Study design. The HFD+L-NAME-induced HFpEF mouse model combines high-fat diet (HFD, 60% kcal fat) and L-NAME (0.5-1.0 g/L in drinking water) administered to 8-week-old C57BL/6 mice for 10-14 weeks to induce metabolic syndrome (obesity, insulin resistance) and hypertension.
Obesity in HFD+L-NAME mice.
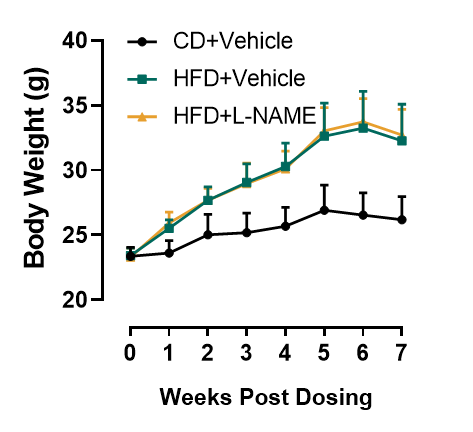
Figure 2. Body weight of HFD+L-NAME induced mice. Similar to mice maintained on HFD (DIO) mice, HFD+L-NAME mice show augmented weight gain and obesity. n=8, Data presented as Mean ± SD.
L-NAME disrupts vasodilation and results hypertension in HFD+L-NAME induction.
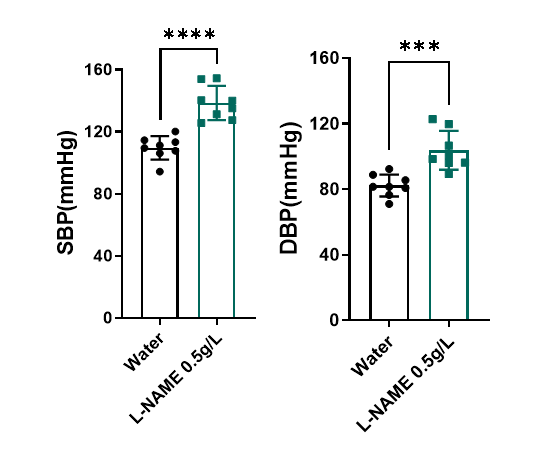
Figure 3. Tail cuff measurement of blood pressure. L-NAME reduces nitric oxide bioavailability, causing hypertension, microvascular inflammation, and impaired vasodilation. One week following L-NAME treatment, systolic blood pressure (SBP) increases over 30mmHg and diastolic blood pressure (DBP) increases over 20mmHg. n=8, Data presented as Mean ± SD.****, p < 0.0001 Vs control mice by t-test.
L-NAME-induced hypertension impairs exercise tolerance.
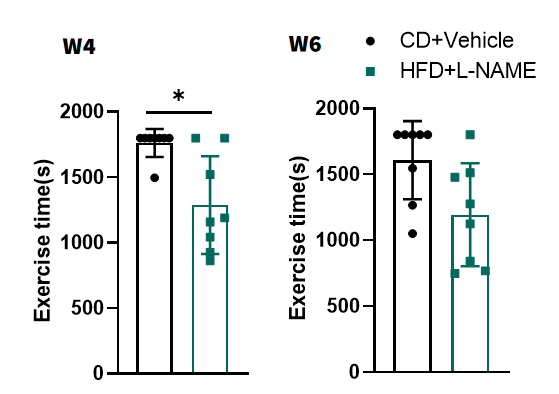
Figure 4. Measurement of exercise tolerance by treadmill. HFD+L-NAME mice showed reduced exhaustion time on treadmill tests, indicating systemic impairment. n=8, Data presented as Mean ± SD. *, p < 0.05 Vs control mice by t-test.
The HFD+L-NAME intervention does not impair systolic function but induces cardiac remodeling.
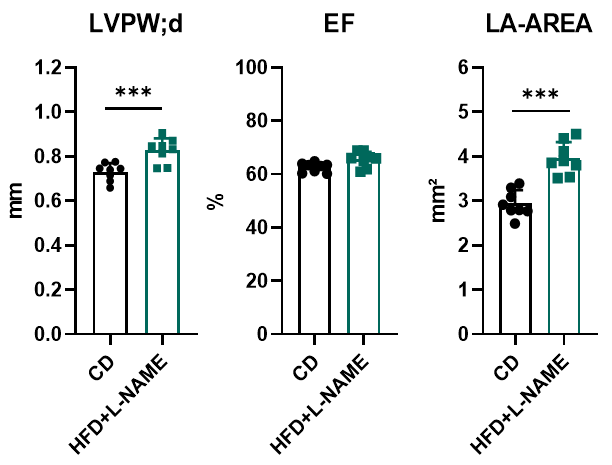
Figure 5. Echocardiography in PSAX. After 10 weeks of HFD+L-NAME treatment, mice developed mild ventricular hypertrophy (evidenced by increased interventricular septum thickness) and left atrial enlargement, while maintaining preserved left ventricular ejection fraction (LVEF). These observations indicate that the HFD+L-NAME "two-hit" model triggers cardiac structural remodeling (e.g., hypertrophy and atrial dilation) without impairing systolic function, consistent with HFpEF pathophysiology characterized by diastolic dysfunction and preserved LVEF. Notably, this aligns with clinical HFpEF features where systolic function remains intact despite metabolic and hemodynamic stress-induced myocardial stiffness and fibrosis.***, p < 0.001 Vs control mice by t-test.
HFD+L-NAME significantly impairs ventricular diastolic function.
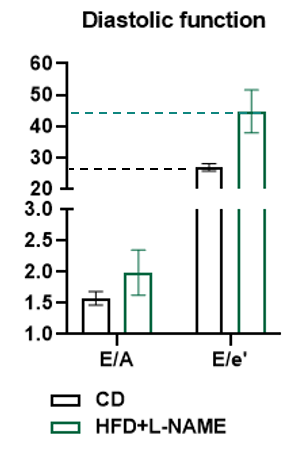
Figure 6. Doppler analysis characterizes diastolic dysfunction in HFD+L-NAMe heart. After 10 weeks of HFD+L-NAME treatment, distinct diastolic impairment is well identified in HFD+L-NAME-treated mice compared to controls (CD). The E/A ratio (early-to-late diastolic filling) showed increase (CD: ~1.5 vs. HFD+L-NAME: ~2.0) and the E/e' ratio (left ventricular filling pressure) surged dramatically (CD: ~20 vs. HFD+L-NAME: ~45), indicating severely elevated ventricular stiffness and impaired relaxation. This aligns with HFpEF pathophysiology, where preserved ejection fraction coexists with diastolic dysfunction driven by metabolic stress and fibrosis. The pronounced E/e' elevation highlights the model’s ability to recapitulate human HFpEF’s hallmark features.